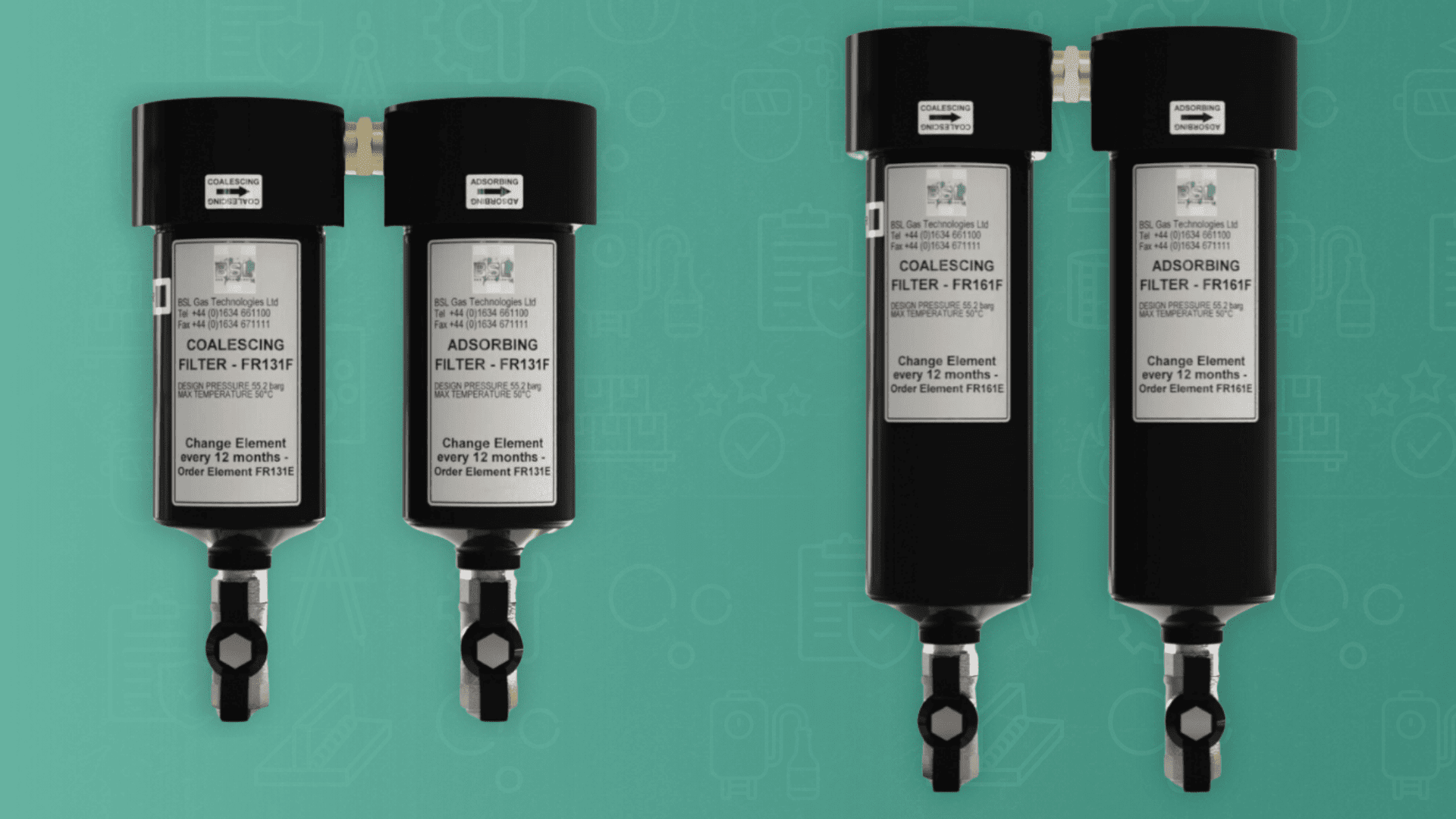
In gas mixing for beverage dispensing, using the right gas blend is vital in preserving the quality and taste of your beverage. Several factors must be considered when determining the correct mix for your beer, such as the as-brewed CO₂ content, cellar storage temperature, and the dispense pressure required for your specific beer.
The as-brewed CO₂ content refers to the level of CO₂ dissolved in the beer during the brewing process. Different styles of beer have different CO₂ contents, and brewers make these choices based on the style they desire. For example:
- Nitro beers: 1.1 to 1.4 volumes
- British beers: 2.2 to 2.4 volumes
- Craft beers and lagers: 2.4 to 2.5 volumes
- General lagers: 2.6 to 2.8 volumes
- European style beers: 3.0 volumes
The as-brewed gas ratios can affect everything about your beverage, from the creaminess of the consistency to waste levels and pour costs.
Another crucial factor is the temperature of the cellar or keg storage facility. While levels may fluctuate, a temperature of 3.3°C or 38°F is considered ideal for carbonated products due to the properties of CO₂ gas in relation to water.
Dispense pressure refers to the force propelling beer from keg to glass, factoring in variables like distance and equipment. The brewer’s specifications and the type of tap and tap head used during dispensing can further dictate this pressure. That’s where BSL comes in.
BSL Cellar-Mix gas mixing panels offer the flexibility to adjust these variables to achieve your desired mix. Whether it’s a low CO₂ mix for nitro beer, a high CO₂ mix for lager, or anything in between, our single BSL Mixing Panels (supplied with CO₂ and N₂) make it happen seamlessly.
What does a typical gas mixing installation look like with BSL?
In a typical installation, CO₂ is stored in a cylinder or liquid mini-bulk supply tank, regulated down to around 5 bar g pressure for beer and safeguarded by pressure relief valves. N₂ gas, on the other hand, is often delivered in a cylinder or nitrogen generator and undergoes a similar process with a regulated pressure of around 6 bar g. Like the CO₂ gas, it will be protected with pressure relief valves.
Our revolutionary Mixer Block is located inside the Gas Mixing Panel, which also houses low pressure outlet pressure regulators and isolation valves. Each outlet also has a further set of two safety pressure relief valves to protect the downstream equipment, including the beer kegs themselves.
The mix is pre-set and tamperproof, but each outlet can be isolated or opened and the outlet pressure can be set for each situation, giving you the freedom and flexibility to control your mix.
Standard panels also come in many configurations; one through to four mixes out, CO₂ outlets for soft drinks at higher pressures, and even N₂ outlets for the dispensing of other products! Installation is simple and the Gas Mixing Panel is at the heart of the process.
What happens to gas pressure in a beer keg?
Inside a keg, gas pressure results from gas molecules bouncing from wall to wall, ricocheting off of the internal surfaces of the container. The force they exert depends on the number and speed of molecules hitting the surfaces.
If a gas-filled keg is heated up, these gas molecules will move faster, hitting the internal surfaces of the keg harder and more frequently, therefore exerting more pressure. Likewise, if that same keg is cooled, the gas molecules will move at a slower rate, hitting the internal surfaces with less force and less frequency, therefore exerting less internal pressure.
To change the internal pressure in the keg, we can change the temperature. We can also change the gas space volume of the keg by changing the number of gas molecules in the container.
Gas Absorption – Under normal beer dispense conditions, gas molecules are constantly moving in and out of solution in the beer. In the gas space above, they hit the surface of the beer and dive into solution, while dissolved gas molecules hit the surface of the solution and break out of it.
Gas Absorption and Pressure – If we increase the gas supply pressure in the keg, the gas molecules will hit the surface of the liquid faster and more frequently than the gas molecules inside the solution.
Initially, more molecules will dive into solution than are breaking out, and this will continue until the molecules have become absorbed to a point of equilibrium; a perfect balance between molecules leaving the solution, and molecules entering it.
Gas Absorption and Temperature – With higher temperatures, the dissolved molecules are able to move faster, hitting the surface with a greater frequency and force. This causes them to break out of the solution.
At lower temperatures, the opposite takes place; more gas molecules remain in the solution (assuming the pressure remains constant). Thus, warmer temperatures require higher pressure levels in order to maintain equilibrium within the keg.
This delicate balance is vital to maintain in order to serve the perfect pint, and BSL Cellar-Mix Panels are designed to maintain it through precise gas blends and pressure regulation.
To find out more, get in touch with us via solutions@bslgastech.com or give us a call on +44(0)1634 66110!
If you’re interested in reading our other blog posts, you can do that here. Make sure you’re following us on LinkedIn, Facebook, Twitter and YouTube so that you never miss an update from us.